Advances and Barriers in Manufacturing for Cell and Gene Therapy
According to our colleagues at Kalorama Information, it is an exciting and interesting time to be involved in the nearly $4 billion cell and gene therapy industry. The science is moving ahead and now the industry needs to industrialize and standardize the manufacturing and commercialization of products. The industry is moving in the direction of a standardized set-up and a closed automated system.
Consequently, cell and gene therapy products are transforming the treatment of cancers and genetic diseases. Additionally, cell and gene therapies are expanding into other areas of medicine including autoimmune diseases, cardiovascular diseases, musculoskeletal disease, dermatological diseases and many others.
Cell and gene therapy manufacturers are forging ahead with more than 1,200 therapies in trials worldwide at present. A review of registered clinical trials databases revealed there are more than 700 investigational cell and gene therapies in clinical development in the US. However, manufacturing facilities have not kept up. It has been estimated that hundreds of facilities will be needed to manufacture the treatments that are now in clinical trials.
One of the areas that needs to be accelerated is viral capacity. The industry is making advancements in this area, but it still has not resolved as the expansion is still falling short of demand. Most viral vectors are produced using adherent manufacturing which are expensive to operate- a vial of 20 million cells can cost $20 to $30 thousand to make.
Manufacturers face many challenges in the development of cell and gene therapies. These challenges occur at different stage of development. In the process development, manufacturers face compressed timelines, limited expertise, lack of manufacturing tools and platforms and numerous dosages that are unique to the target tissue.
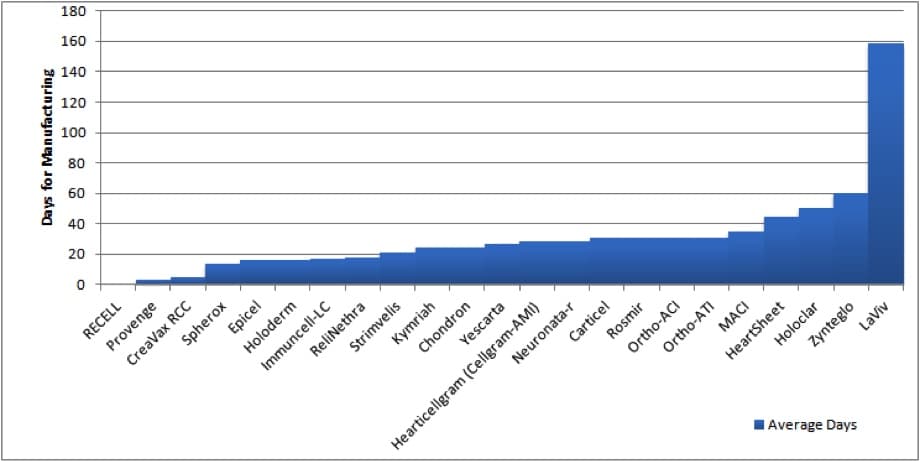
Manufacturing Process Time for Select Autologous Cell and Gene Therapies
In development of safety testing vector characterization, manufacturers face small sample volumes for development of assays, high product titer, significant specificity for confidence, and infinite variations of customized vectors.
In regulatory, manufacturers face lack of standardized guidelines, lack of historical process data, and lack of guidelines around plasmid quality.
Select new manufacturing facilities are listed below including:
- Novartis opened a new manufacturing facility for cell and gene therapies in November 2019 in Stein, Switzerland.
- GE Healthcare (now Cytiva) launched a KUBio box in October 2019 to accelerate the production of viral-vector-based gene therapies and increase capacity in the viral vector space. The new KUBio box features a Germfree biosafety level 2 modular bioprocessing environment and is equipped with the FlexFactory single use biomanufacturing platform, which has been tailored specifically for the production of viral vectors.
- Hitachi Chemical Advanced Therapeutics Solutions opened a new commercial scale cell and gene therapy manufacturing facility in January 2020. The new facility houses six classified environment rooms and has the capacity to add more rooms that can be specifically configured according to need. The facility includes manufacturing and development labs as well as quality control and microbiology labs.
- In August 2019, Pfizer announced that it is investing $500 million to construct a new gene therapy manufacturing facility in Sanford, NC. The facility is expected to support Pfizer’s ongoing investment in gene therapy R&D. The facility would increase Pfizer’s ability to supply both clinical- and commercial-scale quantities of gene therapies, specifically, highly specialized, potentially one-time gene therapies that use custom-made recombinant adeno-associated virus (rAAV) vectors.
- Thermo Fisher Scientific opened its new $90-million viral vector CDMO site in Lexington, MA, in December 2019. The 50,000-ft facility will support development, testing, and manufacture of viral vectors for gene and cell therapies. The new facility adds much-needed capacity for viral vector development and manufacturing, which has been a bottleneck area for biotech companies, according to Thermo Fisher, which has noted that the demand for new gene therapies has outpaced capacity. The investment is expected to accelerate commercialization of new cell and gene therapies by providing a range of services—from drug development through clinical trials to commercial manufacturing.
- Lonza has a dedicated manufacturing facility located in Houston, Texas and is one of the largest cell and gene therapy manufacturing facilities in the world.
Cost is one of the largest issues facing the cell and gene therapy industry. The high cost of producing these new therapies is staggering. For example, after launching Kymriah, Novartis’s price tag was $475,000 and a second product, Yescarta cost $375,000.
Advancements in manufacturing are helping to drive costs down but it is still an expensive process. As with the biologics space with monoclonal antibodies, it has transitioned from a manual process to a more standardized and automated set-up. This is the goal of the cell and gene therapy industry. This will take the industry to a platform that will serve large patient populations while reducing both costs and risks.
Learn more about Kalorama’s report, Cell Therapy and Gene Therapy Markets