Clinical Diagnostics: Rise of Genomics Spurs Innovation in Instruments & Techniques
From Idea to Approval
There are a variety of MDx techniques used in hospitals, research labs, clinics, and commercial or reference labs that are used to detect nucleic acids and proteins for diagnostic or monitoring purposes. Most require an analytical instrument, such as a PCR machine, mass spectrometer, or sequencer. The companies that manufacture these instruments continually look for ways to enhance their detection capabilities and expand the science into new applications. When a new instrument or instrument-based technique hits the market, it generally begins as a clinical or translational research tool designed to study nucleic acids and proteins.
While MDx techniques evolve in different ways and not all methods survive, those that do typically transition from basic research to lab developed tests (LDTs) in applied testing and diagnostic markets, where they are able to be used in a more routine and trusted capacity. Techniques can evolve a step further and achieve approval from country or region specific regulatory agencies — a process that requires rigorous testing to ensure that quality and performance standards are maintained.
The overall MDx market has two major segments, both of which are equally important to the success of the overall program of disease detection and monitoring: clinical research and molecular in vitro diagnostics (IVD). Clinical research involves the development of assays for molecular diagnostics, biomarker discovery, and pharmacogenomic applications. Molecular IVD embodies the analysis and detection of infectious disease, cancer, genetic disorders, metabolic ailments, and others. It represents about one-tenth of the IVD market but is the fastest growing segment within IVD.
Fierce Competition in a Fractured Market
Many of the largest names in life science have already entered the clinical diagnostics space, either with new medical instruments or by acquiring smaller companies fluent in the industry with established product lines.
As recent as November 15th, Illumina introduced their NextSeq ™ 550Dx System, the company’s second FDA-approved and CE-IVD marked NGS system. The company’s MiSeq™ sequencer has also been FDA-approved to include the use of DNA libraries generated from formalin-fixed paraffin embedded (FFPE) tissues, paving the way for clinical labs to use FFPE samples when developing clinical tests for new applications.

Also on November 15th, Fabric Genomics announced a solution for somatic mutations in cancer, which enables clinical labs that perform cancer NGS testing to quickly convert genomic insights to targeted therapies.
In the past two years, Waters, Roche, Thermo Fisher Scientific, Luminex, CombiMatrix, Hologic and several other companies have also received FDA or EU IVD approval for various instruments and techniques in and around the clinical diagnostics space. These approvals cover a wide variety of topics, such as cancer diagnosis and treatment, stillbirth genetic testing, blood testing, virus detection and differentiation, among others.
Navigate Confidently with Trusted Data
With such rapid change in such a quickly growing market, company executives, marketing directors, and product managers at instrument manufacturing companies need a roadmap crafted by the experts. Moving forward with trusted data on hand is critical to take advantage of potential expansion opportunities, partnerships and acquisitions, and for the intelligent allocation of marketing resources.
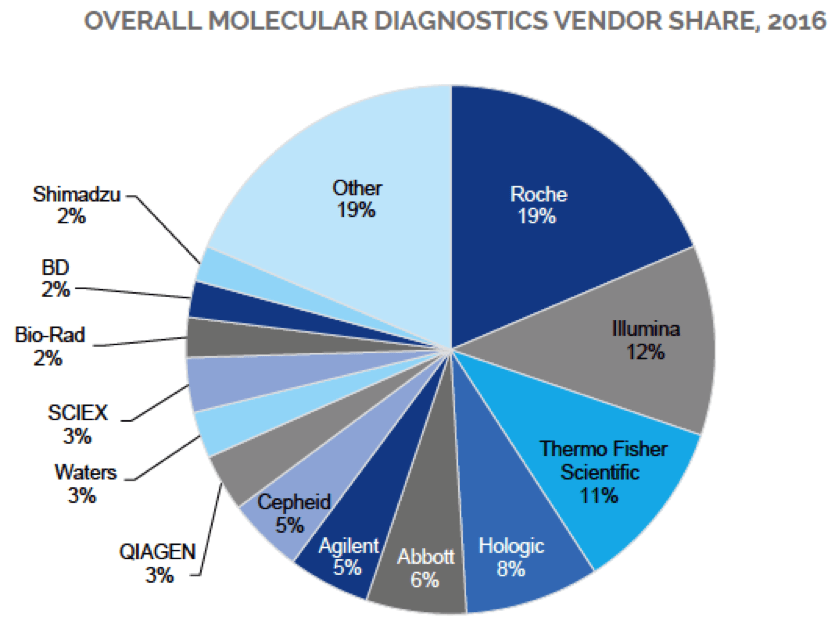
Unlike other market intelligence services, our team of professional analysts and PhD scientists with lab experience both understand the topic and the numbers. In our most recent report, Innovation in Clinical Diagnostics Instruments: PCR, NGS, Mass Spec and HPLC, we provide a roadmap of the MDx market in terms of its current situation, trends, and recent market-oriented data about analytical solutions being used in this field.
This report does not aim to be exhaustive, but it does provide information on techniques where innovation in established technology platforms is most applicable to clinical diagnostics. Thus, the scope of the report is not limited nucleic acid based techniques (amplification, sequencing, microarrays and in situ hybridization), but also includes mass spectrometry and HPLC for the analysis of proteins, hormones, drugs, and other complex, bioactive molecules.
Like many other areas of life science, there is a unique business opportunity for brand growth in the MDx space. But at its core, these innovations will help millions of people worldwide with faster and more accurate diagnosing of disease and genetic conditions, and ultimately better informed recommendations for effective treatments.





