Real-Time PCR: A Mature Market that is Still Evolving
Though a mature market for many years, real-time PCR has still seen growth and advancements in both application as well as throughput efficiency over the past decade, and even in the last few years.
In 2008, QIAGEN’s real-time PCR specific workflow with a standardized solution largely contrasted Thermo Fisher Scientific’s customization and support-based work flow. QIAGEN instead focused on improving their epigenetics workflow.
Despite the recession in 2008 and continued economic stagnation in 2009, real-time PCR systems fueled the nucleic acid amplification market. Multiplexing kits, which improved throughput, continued to drive growth.
A few years later, in 2011, real-time PCR saw more rapid growth as foodborne pathogen testing became pertinent due to microbial contamination of food products as a result of major outbreaks. Real-time PCR assays are still most commonly used for Salmonella, Listeria monocytogenes and E.coli 0157. Salmonella is responsible for 80% of food born outbreaks while Listeria monocytogenes and E.coli 0157 both cause severe illnesses. Raw ingredients, pre-processed foods and processed foods are tested using qPCR, as are processing environments. Bio-Rad Laboratories, Life Technologies (now a Thermo Fisher Company) Roche, BIOTECON, Congen, and DuPont Qualicon were particular contributors to market advancements. Ease of use, speed and validation became particularly important product differentiators.
Idaho Technology won FDA approval for five bacterial respiratory pathogens, bringing their total to 20 viral and bacterial respiratory pathogens in 2012. Additionally, assays based on Seegene’s TOCE- CCMTA real-time PCR technology, covered 21 respiratory viral pathogens, 28 different HPV genotypes, and 7 sexually transmitted pathogens. It is sensitive enough to detect a single point mutation. Over the next two years, real-time PCR played a significant role in single-cell analysis as well as miRNA analysis.
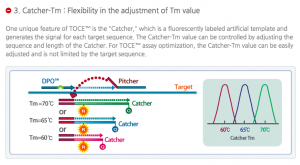
Seegene’s TOCE™ technology enables confirmation of multiple (five or more) target genetic detection and genetic variation. Click to read more.
In 2015, Thermo Fisher’s Applied Biosystems introduced two benchtop real-time PCR instruments that integrated a web browser-based software that allowed data access and analysis anytime and anywhere via the Thermo Fisher Cloud. As gene expression and genotyping has been and continues to be the predominant application of real-time PCR, the nanoliter format was introduced in the late 2000’s to provide the highest sensitivity and widest dynamic range. Now nanoliter formats such as the Smart Chip allow for validation of SNPs as well as drug response monitoring and rare mutation detection.
Continuing to improve and expand detection of sexually transmitted pathogens, SpeeDx and ThermoFisher Scientific announced their combined efforts to detect M. genitalium in 2017. From another collaboration, ACEA Biosciences and Memorial Sloan Kettering Cancer Center’s efforts led to the ability to monitor destruction of cancer cells by a patient’s own genetically modified CAR-T cells.
Real-time PCR is one of the most used methodologies for a wide variety of applications — from quantitation of gene expression, genotyping, infectious disease detection, to food safety testing. Consequently, we should not neglect it despite its status as a mature market, and life science suppliers should maintain product quality, customer satisfaction, and continue to search out ways to innovate.
Competing in a mature market, unlike a growing market, can be difficult as most customers have already established brand loyalty and the rate of growth or innovation stagnates. However, the money companies have initially invested into establishing qPCR products is now paying off in profit. Our Real-Time PCR Interactive Report further illustrates the changes in real-time PCR.





